

The key difference between pure and hybrid orbitals is that pure orbitals are the original atomic orbitals whereas hybrid orbitals form from the mixing of two or more atomic orbitals. In this article, we described two types of orbitals as pure and hybrid orbitals. The below infographic tabulates the difference between pure and hybrid orbitals for quick reference.Ītomic orbitals are the regions where electrons exist in atoms.

When considering the nomenclature of orbitals, we name pure orbitals as s, p, d and f orbitals while we name hybrid orbitals as sp, sp 2, sp 3, etc. Moreover, the formation of hybrid orbitals is important in the formation of complicated chemical compounds via formation of covalent chemical bonds. Furthermore, the hybrid orbitals form via orbital hybridization, but pure orbitals are not hybridized. This is the key difference between pure and hybrid orbitals. Pure orbitals are atomic orbitals that contain electrons of the atom whereas hybrid orbitals are the molecular orbitals that form from the mixing of atomic orbitals. What is the Difference Between Pure and Hybrid Orbitals? We name these orbitals according to the atomic orbitals that undergo hybridization. The process of this mixing is “orbital hybridization” which results in hybrid orbitals. this mixing occurs in order to form a covalent chemical bond with another atom.

The mixing occurs between the atomic orbitals of the same atom. Hybrid orbitals are the molecular orbitals that form from the mixing of atomic orbitals. According to the angular momentum quantum number, there are four commonly known atomic orbitals as s orbital (spherical shaped), p orbital (dumbbell-shaped), d orbital (two dumbbells in the same plane) and f orbital (a complicated structure). Each orbital occupies a maximum of two electrons. This set of numbers include n (principal quantum number), l (angular momentum quantum number), m (magnetic quantum number) and s (spin quantum number). According to the quantum mechanics, there is a set of quantum numbers that we use to name an orbital. The pure atomic orbitals exist in several shapes such as spherical shape, dumbbell shape.
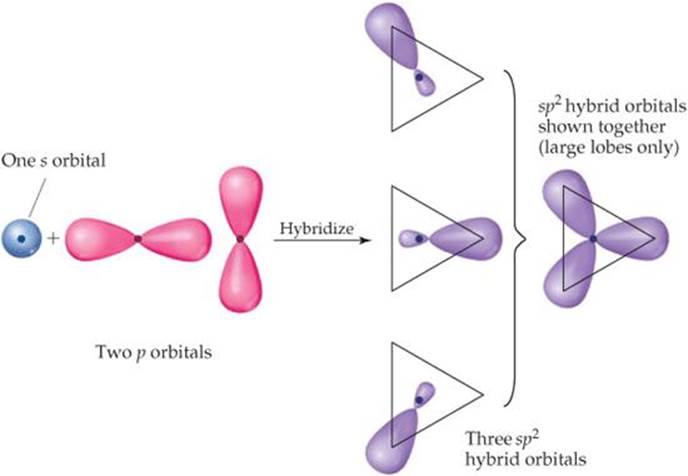
Rather than a fixed location, this gives a region where the electron can occur at a particular time. The orbital gives the most probable location of electrons in an atom since the electrons are in continuous movement around the atomic nucleus. These orbitals are not mixed orbitals like hybrid orbitals. Pure orbitals are atomic orbitals that contain electrons of the atom. Side by Side Comparison – Pure vs Hybrid Orbitals in Tabular Form These orbitals involve in the formation of covalent chemical bonds. Orbital hybridization is the chemical concept that describes the mixing of atomic orbitals to form new hybrid orbitals. But if we are to discuss the chemical bonding in complex molecules, we need to know what is orbital hybridization. In the chemical bond formation of simple molecules, we can simply consider the overlapping of atomic orbitals. The key difference between pure and hybrid orbitals is that the pure orbitals are the original atomic orbitals whereas the hybrid orbitals form from the mixing of two or more atomic orbitals.


 0 kommentar(er)
0 kommentar(er)
